In Acid Base Titration Which Indicator Is Used
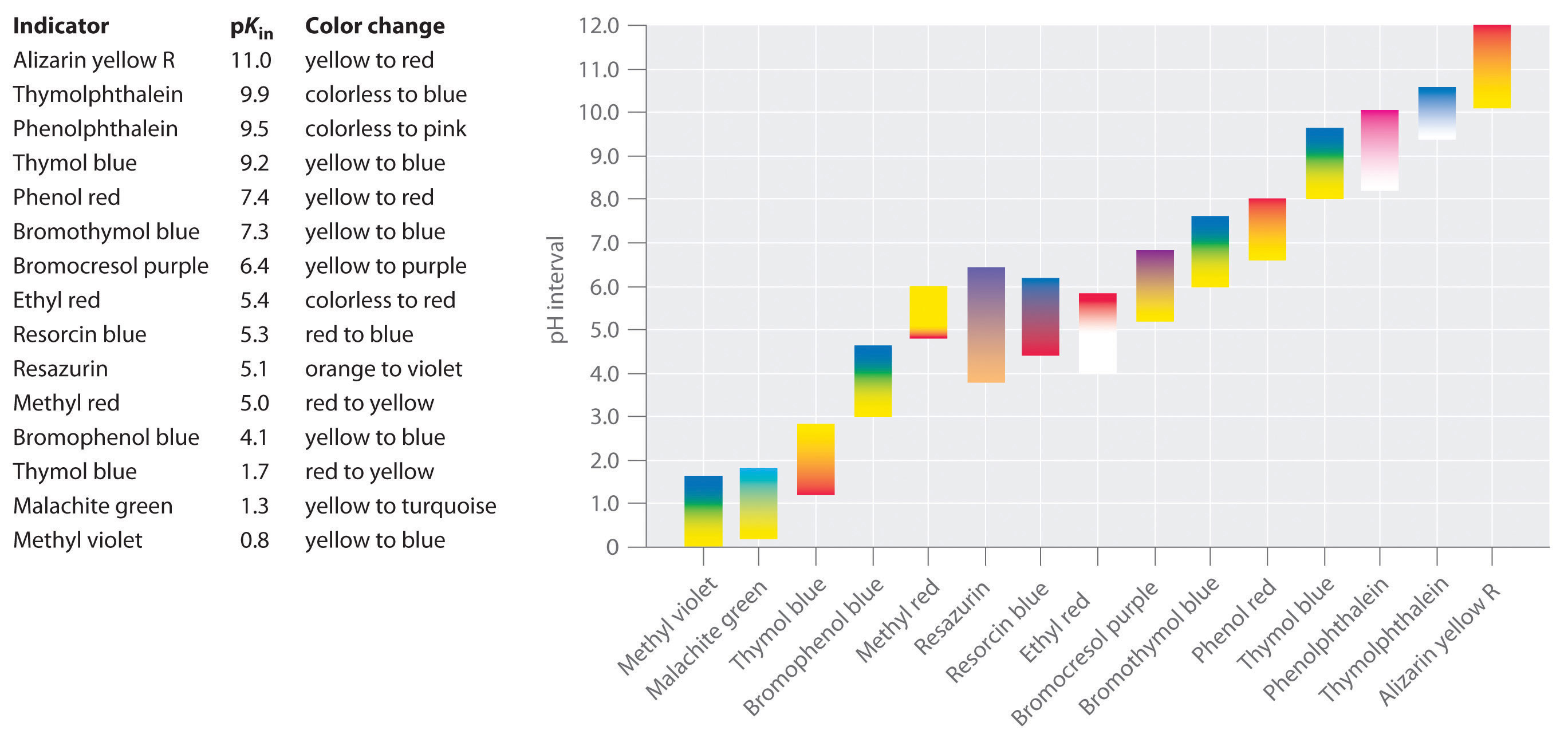
Phenolphtalein was chosen because it changes color at pH levels ranging from 83 to 10. Methyl red and methyl orange.
Pchem Teaching Lab Additional Background Information
Thymol Blue - 2 nd change.
. Adding a proton yields the structure on the right colored red. Strong acid-strong base titration. Phenolphthalein indicator used in acid-base titration.
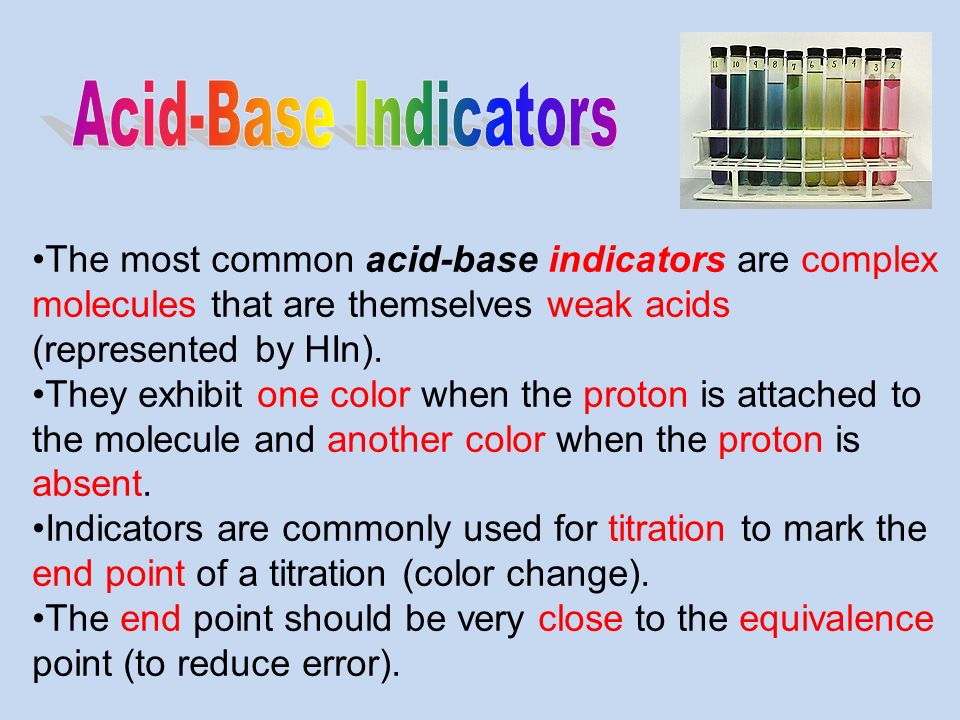
In basic solutions it will look pink. In acid-base titrations the end point is detected by a pH sensitive indicator. Well usually ANY indicator can be used.
Why is phenolphthalein used as an indicator in saponification value of fats. Weak acid-weak base titration. Titration is completed when the solution turns a light pink.
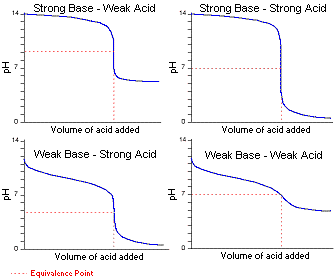
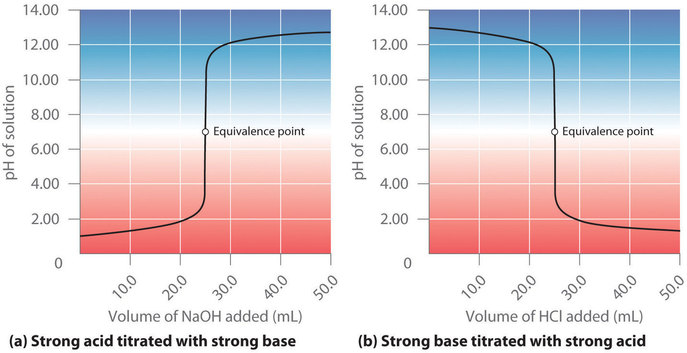
All the indicators show color change at pH 7 D. A suitable indicator for the titration of the weak acid CH3COOHaq and the strong base NaOHaq would be either thymol blue pH range 80 96 or phenolphthalein pH range 83 100. Indicators as the name implies are substances which indicate generally by their color change the equivalence point or end point of a titration.
Indicator showns thend point of acid-base neutralization C. Strong acid-weak base titration. A Methyl orange 3 to 46 b Bromothymol blue 6 to 75 c Phenolphthalein 8 to 96 d Methyl red 5 to 69.
It will appear pink in basic solutions and clear in acidic solutions. Ii Weak acid Vs strong base. In acidic ones it will seem clear.
In this type of titration a drop of an indicator is used at the start which changes its colour to indicate the endpoint. Phenolphthalein is a great indicator when titrating an acid into a base or alkali solution. Methyl orange indicator the base is off the scale eg pH 135 and the acid has pH 55.
Which of the following. The molecule methyl orange is commonly used as an indicator in acid-base equilibrium reactions. Phenolphthalein is slightly soluble in water and usually is dissolved in alcohols for use in experiments.
The indicator turns pink when in the presence of a base. There are different types of indicators used in chemical analysis. A drop of indicator is added in the start of the titration the endpoint has been appeared when color of the solution is changes.
In base form on the left in the figure the color is yellow. If we add acid to a base for titration the indicator should change color at pH around 9. PKa 080 Indicator Acid color Base color Yellow Methyl violet Blue Yellow Cresol red Malachite green Thymol blue Phloxine Orange IV Benzopurpurin Methyl yellow Methyl orange.
In the titration of strong acid and weak base the indicator used is Methyl Orange. Different indicators are used but depend on the strength of an acid and alkali. From the list of acid-base indicators below select the one BEST indicator for a titration with an equivalence point at pH 40 BE CAREFUL-enter indicator name EXACTLY as it appears in table.
For this application it turns colorless in acidic solutions and pink in basic solutions. A strong acid- strong base titration is performed using a phenolphthalein indicator. In the EDTA titration metal ion indicator is used to detect changes of pM.
Titration is based on neutralization. Here an acid or base of known concentration is used to determine the concentration of a given base or acid by neutralisation. We can use the same indicator for all the acid-base titrations B.
Phenolphthalein is chosen because it changes color in a pH range between 83 10. Phenolphtalein is chosen because it changes color in a pH range between 83 10. The suitable indicators for the following titrations are i Strong acid Vs strong base.
It is the negative logarithm of the free metal ion concentration ie pM - log M 2. In an acid-base titration an indicator is used. The acid-base titration involves a neutralisation reaction.
Iii Strong acid Vs weak base. The Universal Indicator Color Guide shows that Universal Indicator turns red when it is added to a strong acid it turns purple when it is added to a strong base and it turns a yellowish-green when it is added to a neutral solution. Phenolphthalein pH range 83 to 105 methyl red pH range 44 65 and methyl orange pH range 32 to 45.
Thymol Blue - 1 st change. A phenolphthalein indicator is used to do a strong acid-strong base titration. Indicators in Acid-Base Titrations.
Redox indicators are also used which undergo change in color at specific electrode potential 2. Phenophthalein is an appropriate indicator for titration of weak acids with strong bases as the end point will be in same pH range. Can you use universal indicator for titration.
The indicator changes the colour with the change in the pH of the solution. Phenophthalein would not be appropriate for stong acid-strong base titrations as the end point would be at pH 7. Which of the following is used as an indicator in the titration of a weak acid and a strong base.
Phenolphthalein is often used as an indicator in acidbase titrations. Phenolphthalein a commonly used indicator in acid and base titration. Note that this color change occurs over the pH range from approximately 3-4.
The two common indicators used in acid-base titration is Phenolphthalein and methyl orange. It will appear pink in basic solutions and clear in acidic solutions. Answer 1 of 2.
On the other hand with weak acidstrong base titrations the indica. Universal indicator which is actually a mixture of several indicators displays a variety of colours over a wide pH range so it can be used to determine an approximate pH of a solution but is not used for titrations. 12 rows Not all acid-base indicators are suitable for use in acid-base titrations.
What indicator would you use to titrate HCL and NaOH.
7 3 Acid Base Titrations Chemistry Libretexts

Acid Base Indicator Definition Concept Video Lesson Transcript Study Com

Acid Base Titrations Chemistry For Majors

Acid Base Titration Titration Curves Equivalence Point Indicators Of Acid Base Titration

Indicators Titration Mcat Content
Acid Base Indicators The Most Common Acid Base Indicators Are Complex Molecules That Are Themselves Weak Acids Represented By Hin They Exhibit One Color Ppt Video Online Download
16 5 Acid Base Titrations Chemistry Libretexts

Acid Base Indicators The Most Common Acid Base Indicators Are Complex Molecules That Are Themselves Weak Acids Represented By Hin They Exhibit One Color Ppt Video Online Download
What Does The Choice Of Indicator In Acid Base Titration Depend On Quora
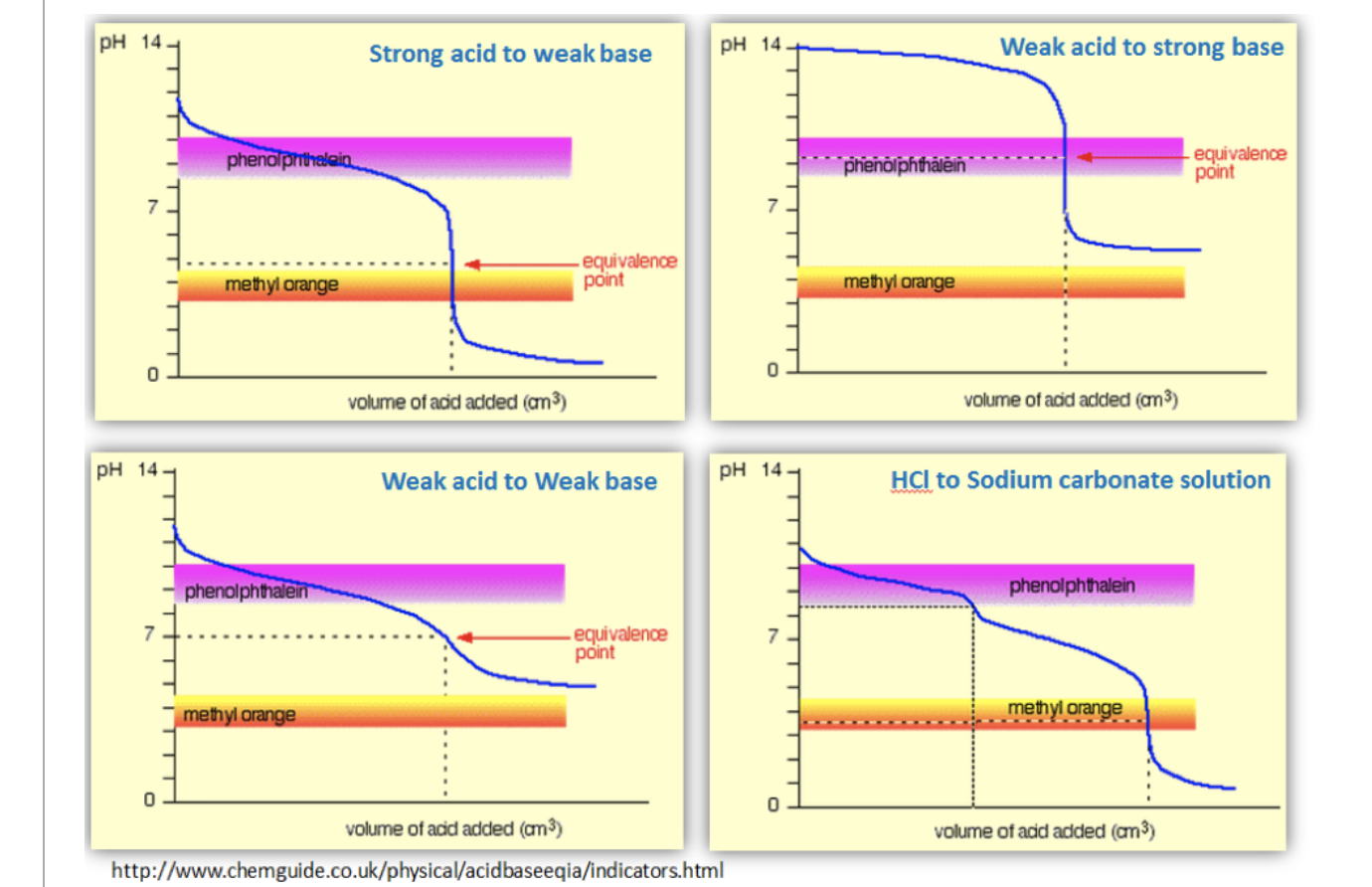
Solved 1 Figure 1 Shows The Titration Curves Of Four Chegg Com
What Indicator Is Used In Strong Acid And Weak Base Titration And Why Quora
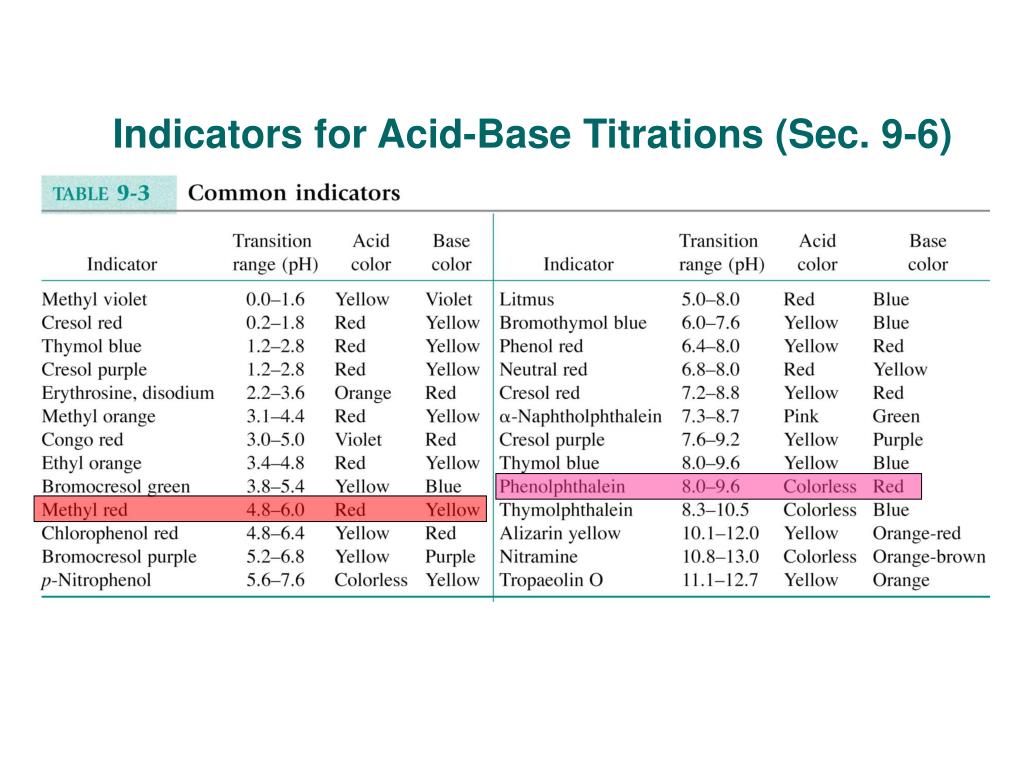
Ppt Indicators For Acid Base Titrations Sec 9 6 Powerpoint Presentation Id 4250069
Which Indicator Is Used In A Strong Acid Versus A Strong Base Solution Quora

Part 23 Indicators Of Acid Base Titrations Theories Of Indicators Acid Base Titrations Youtube
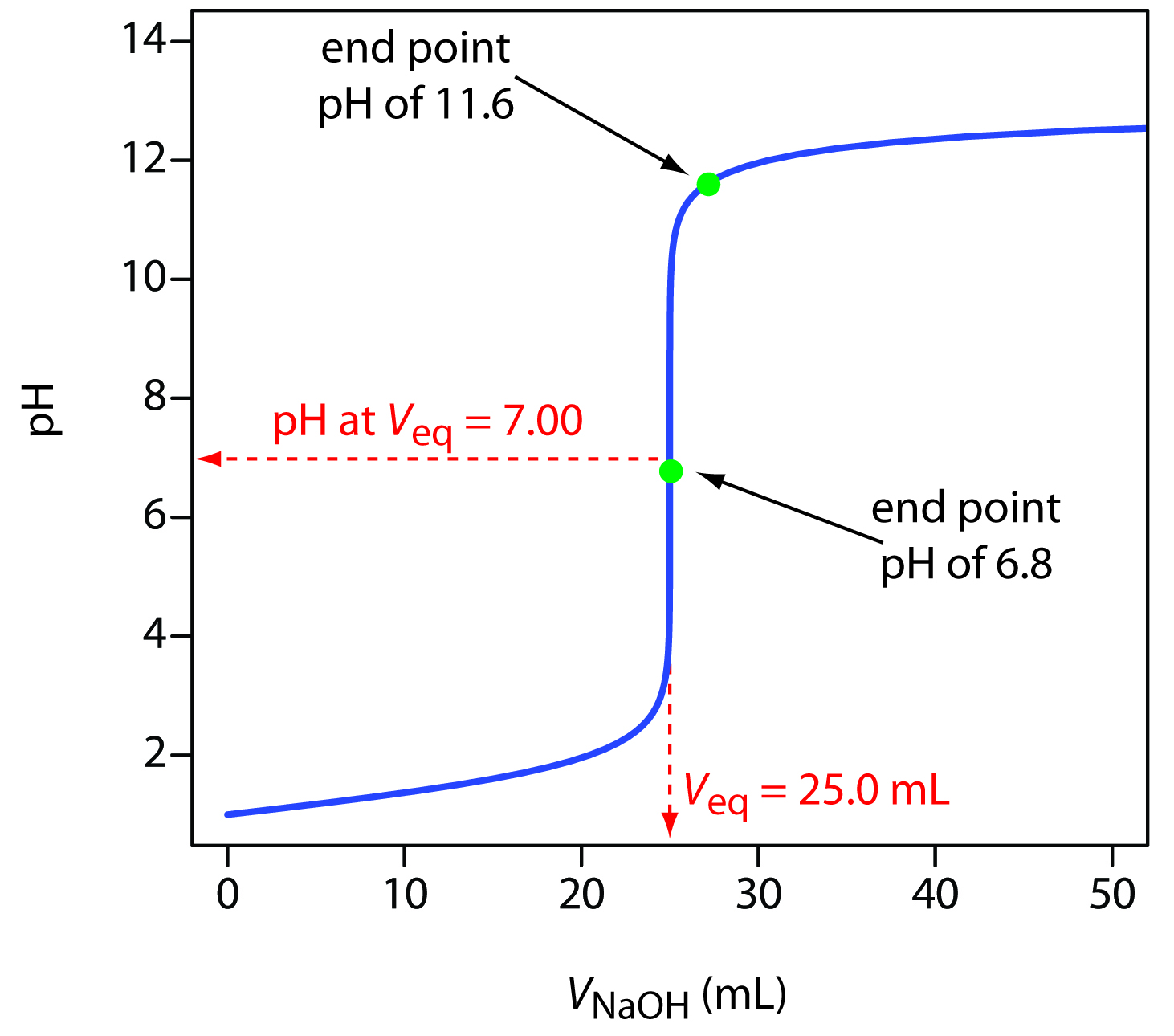
Why Do We Use Indicators In Acid Base Titrations Socratic

Indicators For Acid Base Knowledge Of Science By Shb Facebook

Theory Of Acid Base Indicators And Acid Base Titration Curves
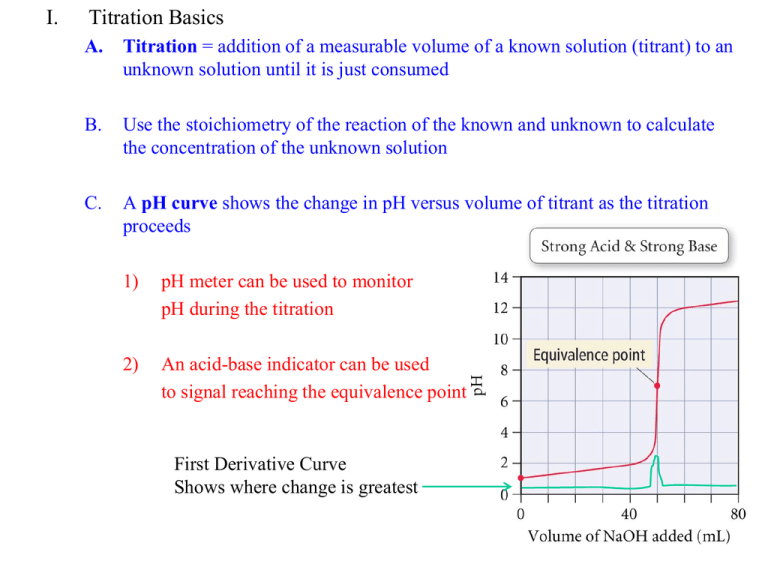

Comments
Post a Comment